Controlling pH - adding calcium...
- Shaun
- Posts: 229
- Joined: 07 Jul 2006, 02:45
- My cats species list: 12 (i:0, k:0)
- Location 2: Australia
- Interests: Catfish...
Controlling pH - adding calcium...
Tapwater in the area I've recently moved to is rainwater, it comes out of the tap 7.5+ due to the addition of alkaline chloromine, but after only hours in a tank with driftwood, fish waste, nitrogen etc it will crash to lower then my liquid test kits can read, around 5.5ish.
I'm not a huge fan of adding chemicals/additives, I just use a little salt with my water changes. What if I add some calcium rich substances such as coral sand, coral gravel or white rock. I'll have to experiment to where I can get pH around 7, but I'm hoping this will stabalise my water?
Drawbacks could be adding too much and sending pH too high?
Thanks,
Shaun
I'm not a huge fan of adding chemicals/additives, I just use a little salt with my water changes. What if I add some calcium rich substances such as coral sand, coral gravel or white rock. I'll have to experiment to where I can get pH around 7, but I'm hoping this will stabalise my water?
Drawbacks could be adding too much and sending pH too high?
Thanks,
Shaun
- MatsP
- Posts: 21038
- Joined: 06 Oct 2004, 13:58
- My articles: 4
- My images: 28
- My cats species list: 117 (i:33, k:0)
- My aquaria list: 12 (i:10)
- My BLogs: 4 (i:0, p:97)
- Spotted: 187
- Location 1: North of Cambridge
- Location 2: England.
Re: Controlling pH - adding calcium...
It would probably be better to add a tiny bit of Sodium bicarbonate (or if you have plants, Potassium bicarbonate). Sodium bicarbonate is the same as "Baking soda" used in home baking [but NOT the same as Baking powder, which has other stuff added to it, as well as Sodium bicarbonate].
You would want to have a KH test to see where you are going to.
--
Mats
You would want to have a KH test to see where you are going to.
--
Mats
- Shaun
- Posts: 229
- Joined: 07 Jul 2006, 02:45
- My cats species list: 12 (i:0, k:0)
- Location 2: Australia
- Interests: Catfish...
Re: Controlling pH - adding calcium...
I thought Baking Soda would raise pH but not buffer alkalinity? I've got plenty of PH Up, which is essentially just Baking Soda, but I didn't want to use it for fear of pH crash.
Anyway, I've added a small amount of coral gravel to the substrate and the pH has raised from 6 to 7. Quicker then I would've liked to do it, but thats what was happening with every waterchange anyway, and then it would drop back down in less then a day. It seems stable so far, was 7 before and after a waterchange this afternoon.
Shaun
Anyway, I've added a small amount of coral gravel to the substrate and the pH has raised from 6 to 7. Quicker then I would've liked to do it, but thats what was happening with every waterchange anyway, and then it would drop back down in less then a day. It seems stable so far, was 7 before and after a waterchange this afternoon.
Shaun
- racoll
- Posts: 5258
- Joined: 26 Jan 2004, 12:18
- My articles: 6
- My images: 182
- My catfish: 2
- My cats species list: 2 (i:2, k:0)
- My aquaria list: 1 (i:0)
- Spotted: 238
- Location 1: London
- Location 2: UK
Re: Controlling pH - adding calcium...
Alkalinity is what increases pH and buffers the water.Shaun wrote:I thought Baking Soda would raise pH but not buffer alkalinity?
- TwoTankAmin
- Posts: 1491
- Joined: 24 Apr 2008, 23:26
- I've donated: $4438.00!
- My cats species list: 6 (i:0, k:0)
- My BLogs: 2 (i:0, p:48)
- Location 1: USA
- Location 2: Mt. Kisco, NY
- Interests: Fish and Poker
Re: Controlling pH - adding calcium...
From thekrib.com via their mirror site http://fins.actwin.com/aquariafaq.html
The following measurements are approximate; use a test kit to verify you've achieved the intended results. Note that if your water is extremely soft to begin with (1 degree KH or less), you may get a drastic change in pH as the buffer is added.
To raise both GH and KH simultaneously, add calcium carbonate (CaCO3). 1/2 teaspoon per 100 liters of water will increase both the KH and GH by about 1-2 dH. Alternatively, add some sea shells, coral, limestone, marble chips, etc. to your filter.
To raise the KH without raising the GH, add sodium bicarbonate (NaHCO3), commonly known as baking soda. 1/2 teaspoon per 100 Liters raises the KH by about 1 dH. Sodium bicarbonate drives the pH towards an equilibrium value of 8.2.
“No one has ever become poor by giving.” Anonymous
“Everyone is entitled to his own opinion, but not to his own facts.”" Daniel Patrick Moynihan
"The good thing about science is that it’s true whether or not you believe in it." Neil DeGrasse Tyson
“Everyone is entitled to his own opinion, but not to his own facts.”" Daniel Patrick Moynihan
"The good thing about science is that it’s true whether or not you believe in it." Neil DeGrasse Tyson
- apistomaster
- Posts: 4735
- Joined: 10 Jun 2006, 14:26
- I've donated: $90.00!
- My articles: 1
- My cats species list: 12 (i:0, k:0)
- My Wishlist: 1
- Location 1: Clarkston, WA, USA
- Location 2: Clarkston, WA, USA
- Interests: Aquaculture and flyfishing
Re: Controlling pH - adding calcium...
Hi twotankamin,
Since your water is already suitable for breeding any of the small pleco species including H. zebra, why is increasing the pH and hardness so important to you?
Just skipping some water changes then stepping them back up is much simpler and would work just as well.
It is normal to experience an occasional break among groups of routinely spawning plecos no matter how you breed them.
There is so much empirical evidence in support of the relatively low importance of water pH and hardness in breeding all the small plecos. Small changes in the hardness are not nearly as important as keeping up with the water changes unless your water is very hard and has a high pH.
I only trying to learn why and understand what makes you think these changes you are trying are so much more important than a good diet and your already excellent water chemistry.
Since your water is already suitable for breeding any of the small pleco species including H. zebra, why is increasing the pH and hardness so important to you?
Just skipping some water changes then stepping them back up is much simpler and would work just as well.
It is normal to experience an occasional break among groups of routinely spawning plecos no matter how you breed them.
There is so much empirical evidence in support of the relatively low importance of water pH and hardness in breeding all the small plecos. Small changes in the hardness are not nearly as important as keeping up with the water changes unless your water is very hard and has a high pH.
I only trying to learn why and understand what makes you think these changes you are trying are so much more important than a good diet and your already excellent water chemistry.
Avid Trout fly fisherman. ·´¯`·...¸><)))º>
- Shaun
- Posts: 229
- Joined: 07 Jul 2006, 02:45
- My cats species list: 12 (i:0, k:0)
- Location 2: Australia
- Interests: Catfish...
Re: Controlling pH - adding calcium...
In my case I'm just trying to control unstable water that I believe is preventing my fish from breeding. I used to have great tapwater 
Shaun
Shaun
- apistomaster
- Posts: 4735
- Joined: 10 Jun 2006, 14:26
- I've donated: $90.00!
- My articles: 1
- My cats species list: 12 (i:0, k:0)
- My Wishlist: 1
- Location 1: Clarkston, WA, USA
- Location 2: Clarkston, WA, USA
- Interests: Aquaculture and flyfishing
Re: Controlling pH - adding calcium...
If my tap water varied a lot I would give up and strictly adjust RO water to my preferred water chemistry range.
Avid Trout fly fisherman. ·´¯`·...¸><)))º>
- TwoTankAmin
- Posts: 1491
- Joined: 24 Apr 2008, 23:26
- I've donated: $4438.00!
- My cats species list: 6 (i:0, k:0)
- My BLogs: 2 (i:0, p:48)
- Location 1: USA
- Location 2: Mt. Kisco, NY
- Interests: Fish and Poker
Re: Controlling pH - adding calcium...
Larry-
I think I covered my own issues in my original post which I assumed was settled in that thread here http://www.planetcatfish.com/forum/view ... =5&t=33073. Also I was never interested in changing my pH.
In this thread all I was posting was an answer to the OP's original question. I expressed no opinions at all?
But I think both Shaun and I would be interested to know exactly why it is such a big deal to choose one of the two possible and practical ways for dealing with these sort of issues. From purely economical, space and time spent considerations it seems to me raising ones tds with additives and then using one's tap water to lower them sure beats having to use RO and RO additives. Both methods will work if the goal is to create dry and rainy seasons.
In my case there is simply is no space for me to handle RO production and storage. I can't speak to this consideration for Shaun.
Chris
I think I covered my own issues in my original post which I assumed was settled in that thread here http://www.planetcatfish.com/forum/view ... =5&t=33073. Also I was never interested in changing my pH.
In this thread all I was posting was an answer to the OP's original question. I expressed no opinions at all?
But I think both Shaun and I would be interested to know exactly why it is such a big deal to choose one of the two possible and practical ways for dealing with these sort of issues. From purely economical, space and time spent considerations it seems to me raising ones tds with additives and then using one's tap water to lower them sure beats having to use RO and RO additives. Both methods will work if the goal is to create dry and rainy seasons.
In my case there is simply is no space for me to handle RO production and storage. I can't speak to this consideration for Shaun.
Chris
“No one has ever become poor by giving.” Anonymous
“Everyone is entitled to his own opinion, but not to his own facts.”" Daniel Patrick Moynihan
"The good thing about science is that it’s true whether or not you believe in it." Neil DeGrasse Tyson
“Everyone is entitled to his own opinion, but not to his own facts.”" Daniel Patrick Moynihan
"The good thing about science is that it’s true whether or not you believe in it." Neil DeGrasse Tyson
- MatsP
- Posts: 21038
- Joined: 06 Oct 2004, 13:58
- My articles: 4
- My images: 28
- My cats species list: 117 (i:33, k:0)
- My aquaria list: 12 (i:10)
- My BLogs: 4 (i:0, p:97)
- Spotted: 187
- Location 1: North of Cambridge
- Location 2: England.
Re: Controlling pH - adding calcium...
I haven't suggested RO usage, and if the water is SOFT and otherwise problem free [that is, no toxic content, low in nitrate, etc], then I agree, RO is not needed.
If the water is inconsistent, then RO is one option to solve the inconsistency, by removing the components in the tap-water that cause the changes in water "quality". Alternatively, you need to measure what the water is like for every water change, and make suitable adjustments.
--
Mats
If the water is inconsistent, then RO is one option to solve the inconsistency, by removing the components in the tap-water that cause the changes in water "quality". Alternatively, you need to measure what the water is like for every water change, and make suitable adjustments.
--
Mats
- Shaun
- Posts: 229
- Joined: 07 Jul 2006, 02:45
- My cats species list: 12 (i:0, k:0)
- Location 2: Australia
- Interests: Catfish...
Re: Controlling pH - adding calcium...
The water is extremely soft, registers nothing on the liquid test kits I have for GH and KH. I don't have a TDS meter but I've been meaning to take a sample into work and test TDS there. It comes out of the tap with an alkaline pH due to the chloramine added by the water company, but this drops very quickly once dechlorinator (prime) is added. I guess the driftwood/fish waste in the tanks lowers the pH. I do regular water changes 25-50% weekly.
I just want to stabalise the pH so it's not swinging after every water change. So far the small amount of coral gravel I've added seems to have it set at 7.
Shaun
I just want to stabalise the pH so it's not swinging after every water change. So far the small amount of coral gravel I've added seems to have it set at 7.
Shaun
- racoll
- Posts: 5258
- Joined: 26 Jan 2004, 12:18
- My articles: 6
- My images: 182
- My catfish: 2
- My cats species list: 2 (i:2, k:0)
- My aquaria list: 1 (i:0)
- Spotted: 238
- Location 1: London
- Location 2: UK
Re: Controlling pH - adding calcium...
So what is the problem with using the coral buffer in the tank, and then changing water as usual? Are the fishes reacting badly? Most riverine fishes will be fine with small variations.
There are a few options:
1) only keep fishes that are happy in low pH water and use straight tapwater
2) continue to use the coral buffer in the tank, but make smaller, more regular water changes to keep things more stable.
3) try to use a buffer such as sodium bicarbonate (or proprietary product) to get the tapwater to a the pH you want. This will require careful monitoring though, as overdosing could easily raise pH too high.
There are a few options:
1) only keep fishes that are happy in low pH water and use straight tapwater
2) continue to use the coral buffer in the tank, but make smaller, more regular water changes to keep things more stable.
3) try to use a buffer such as sodium bicarbonate (or proprietary product) to get the tapwater to a the pH you want. This will require careful monitoring though, as overdosing could easily raise pH too high.
- MatsP
- Posts: 21038
- Joined: 06 Oct 2004, 13:58
- My articles: 4
- My images: 28
- My cats species list: 117 (i:33, k:0)
- My aquaria list: 12 (i:10)
- My BLogs: 4 (i:0, p:97)
- Spotted: 187
- Location 1: North of Cambridge
- Location 2: England.
Re: Controlling pH - adding calcium...
Can I first point out that it's not calcium as such that makes the water stable - it's the bicarbonate that forms when the calciumCARBONATE is dissolving in the water. So it's not VERY important whether it is baking soda or crushed coral - the effect will be roughly the same. The difference is that adding calcium carbonate in the form of a powder is difficult, because it doesn't dissolve well in water. Sodium bicarbonate does.
The pH drops as a consequence of the carbonate being used up by the nitrogen cycle, as well as tannins leeching from the wood.
--
Mats
The pH drops as a consequence of the carbonate being used up by the nitrogen cycle, as well as tannins leeching from the wood.
--
Mats
- apistomaster
- Posts: 4735
- Joined: 10 Jun 2006, 14:26
- I've donated: $90.00!
- My articles: 1
- My cats species list: 12 (i:0, k:0)
- My Wishlist: 1
- Location 1: Clarkston, WA, USA
- Location 2: Clarkston, WA, USA
- Interests: Aquaculture and flyfishing
Re: Controlling pH - adding calcium...
As lover of mainly species that prefer soft, acid water this is one "problem" I would love to have.
I do not just like catfish; I like breeding wild Discus, Tetras, Pencilfish, South American Dwarf Cichlids, Killiefish and West African Killiefish and the SE Asian small red Betta species and Boraras species. All of these are so much easier to breed in soft, acid water.
I have to make all my soft acid water using an RO unit and even the most efficient RO units produce a small amount of usable water compared to the mineral enriched "waste" water.
I do not just like catfish; I like breeding wild Discus, Tetras, Pencilfish, South American Dwarf Cichlids, Killiefish and West African Killiefish and the SE Asian small red Betta species and Boraras species. All of these are so much easier to breed in soft, acid water.
I have to make all my soft acid water using an RO unit and even the most efficient RO units produce a small amount of usable water compared to the mineral enriched "waste" water.
Avid Trout fly fisherman. ·´¯`·...¸><)))º>
- Shaun
- Posts: 229
- Joined: 07 Jul 2006, 02:45
- My cats species list: 12 (i:0, k:0)
- Location 2: Australia
- Interests: Catfish...
Re: Controlling pH - adding calcium...
Most of the fish I keep will prefer soft, slightly acidic water as well, but they will also appreciate stability, and that's what my tanks don't have at the moment. The pH stabalises far too low (my test kit goes down to 5.5 and it's lower then that), and with every waterchange it goes up and down in the space of a few hours. This can't be good for the fish?
If I was breeding blackwater killifish or Discus then I'd probably not worry too much. At my old place ALL my fish were breeding, water out of the tap was GH 100 and pH was 6.8-7.2 always. I was breeding numerous Corydoras, Bristlenose, L002, L134, Hoplos and Zamora catfish producing fertile eggs that hatched. I was also breeding Golden Pencilfish, Lemon Tetras and Guppies. Since moving, pretty much everything but the Pencilfish and Bristlenose have stopped.
Shaun
If I was breeding blackwater killifish or Discus then I'd probably not worry too much. At my old place ALL my fish were breeding, water out of the tap was GH 100 and pH was 6.8-7.2 always. I was breeding numerous Corydoras, Bristlenose, L002, L134, Hoplos and Zamora catfish producing fertile eggs that hatched. I was also breeding Golden Pencilfish, Lemon Tetras and Guppies. Since moving, pretty much everything but the Pencilfish and Bristlenose have stopped.
Shaun
- MatsP
- Posts: 21038
- Joined: 06 Oct 2004, 13:58
- My articles: 4
- My images: 28
- My cats species list: 117 (i:33, k:0)
- My aquaria list: 12 (i:10)
- My BLogs: 4 (i:0, p:97)
- Spotted: 187
- Location 1: North of Cambridge
- Location 2: England.
Re: Controlling pH - adding calcium...
I'm not at all convinced that pH stability is as important as people say - clearly we don't want to vary 4 pH levels between one water change and the next. But in a general sense, pH [in nature, where the water isn't "messed with" by water companies, etc] is just another way to measure the conductivity - and keeping the conductivity relatively stable.
pH under about 5 is indeed not great for the filter system. So adding a small amount of KH buffer (baking soda is the easiest to get hold of and use if you don't want to pay $$$ for aquarium shop equivalents [that contain the same thing]).
--
Mats
pH under about 5 is indeed not great for the filter system. So adding a small amount of KH buffer (baking soda is the easiest to get hold of and use if you don't want to pay $$$ for aquarium shop equivalents [that contain the same thing]).
--
Mats
-
dw1305
- Posts: 1096
- Joined: 22 Oct 2009, 11:57
- Location 1: Corsham, UK
- Location 2: Bath, UK
- Interests: Natural History, Ecology, Plants, Biotopes, Taxonomy, Nitrification, Cricket & Northern Soul
Re: Controlling pH - adding calcium...
Hi all,
I also agree with what the others have said, in this case it is the mechanism that we use to "measure" acidity/alkalinity that is the problem, not the water. The problem is that the pH scale is a totally meaningless measurement if you have pure water, that is water (a solvent) with very few salts (the solutes) in it. The pH will go rapidly up and down as the ratio of acids (H+ donors) and alkalis (also called bases) (H+ acceptors) change, but this doesn't damage soft-water fish, it is something that happens to them every day in the wild (as the ratios of CO2 and O2 in the water change due to photosynthesis and respiration).
A good way to think of this is as a set of scales, when you are pH7 the scales are balanced and the acid and alkali ends of the scales have the same mass in them, but it doesn't matter what that mass is, pH7 could be because both ends of the scale have 2 grains of sugar in them, or a 10 kilo sack of sugar in each scale.
In that scenario if we add another 2 grains of salt to the "acid" end of the "low" scale it will tip towards the heavy end, as it is 100% increase in mass and change in the ratio from 1:1 to 2:1 "acid:alkali", if we add 2 grains to either side of the 10 kg scale it will not change the ratio of "acid:alkali", or pH, at all.
In this case the weights can be thought of as both the dKH, the carbonate buffering and as the amount of solutes in the water, the conductivity or TDS. That is why measuring these tells you much more than the pH does.
This is the "scales" for highly buffered, base rich alkaline water:
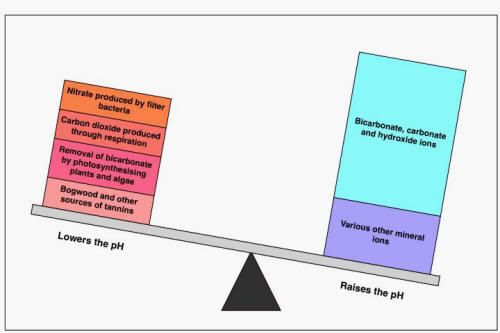
cheers Darrel
I think a small amount of potassium carbonate (or baking soda), oyster shell grit or coral sand should be fine.I just want to stabilise the pH so it's not swinging after every water change. So far the small amount of coral gravel I've added seems to have it set at pH 7.
I also agree with what the others have said, in this case it is the mechanism that we use to "measure" acidity/alkalinity that is the problem, not the water. The problem is that the pH scale is a totally meaningless measurement if you have pure water, that is water (a solvent) with very few salts (the solutes) in it. The pH will go rapidly up and down as the ratio of acids (H+ donors) and alkalis (also called bases) (H+ acceptors) change, but this doesn't damage soft-water fish, it is something that happens to them every day in the wild (as the ratios of CO2 and O2 in the water change due to photosynthesis and respiration).
A good way to think of this is as a set of scales, when you are pH7 the scales are balanced and the acid and alkali ends of the scales have the same mass in them, but it doesn't matter what that mass is, pH7 could be because both ends of the scale have 2 grains of sugar in them, or a 10 kilo sack of sugar in each scale.
In that scenario if we add another 2 grains of salt to the "acid" end of the "low" scale it will tip towards the heavy end, as it is 100% increase in mass and change in the ratio from 1:1 to 2:1 "acid:alkali", if we add 2 grains to either side of the 10 kg scale it will not change the ratio of "acid:alkali", or pH, at all.
In this case the weights can be thought of as both the dKH, the carbonate buffering and as the amount of solutes in the water, the conductivity or TDS. That is why measuring these tells you much more than the pH does.
This is the "scales" for highly buffered, base rich alkaline water:

cheers Darrel
- apistomaster
- Posts: 4735
- Joined: 10 Jun 2006, 14:26
- I've donated: $90.00!
- My articles: 1
- My cats species list: 12 (i:0, k:0)
- My Wishlist: 1
- Location 1: Clarkston, WA, USA
- Location 2: Clarkston, WA, USA
- Interests: Aquaculture and flyfishing
Re: Controlling pH - adding calcium...
Darrel,
Your post was excellent.
Water chemistry is so simple in some ways and so very complex in others. If one doesn't have at least a meager understanding of chemistry many aspects they believe are important are not and many things important are difficult for hobbyists to understand.
The simple facts of the natural fluctuations in an aquatic habitat between night and day are often greater than the minor changes some hobbyists believe are so important in their quest to find which factors influence fishes breeding requirements. There is so much more than minor changes to water chemistry to get fish to breed.
Hobbyists often cause themselves more problems tweaking water chemistry and are better off being more concerned with water quality regardless of their water's chemistry.
I have bred and raised wild Discus in water much harder and more alkaline than many hobbyists have read is possible for just keeping them. Then there are always some species which are so inherently difficult to breed in captivity, Heckel Discus, for example, that there is virtually nothing most of us can do to make it any easier.
When only a handful of people over the past 50 years have managed to breed them, the reasons why are surely more complex than the exact pH and hardness of their water.
I have had similar experiences with all the plecos I have bred.
It can be paradoxical at times because if you do understand the basics of aquarium water chemistry you can get better results by making up your own designer water but unless you have a system or the knowledge which allows you to keep your water chemistry predictable, you are usually better off not playing around with the water's chemistry.
Your post was excellent.
Water chemistry is so simple in some ways and so very complex in others. If one doesn't have at least a meager understanding of chemistry many aspects they believe are important are not and many things important are difficult for hobbyists to understand.
The simple facts of the natural fluctuations in an aquatic habitat between night and day are often greater than the minor changes some hobbyists believe are so important in their quest to find which factors influence fishes breeding requirements. There is so much more than minor changes to water chemistry to get fish to breed.
Hobbyists often cause themselves more problems tweaking water chemistry and are better off being more concerned with water quality regardless of their water's chemistry.
I have bred and raised wild Discus in water much harder and more alkaline than many hobbyists have read is possible for just keeping them. Then there are always some species which are so inherently difficult to breed in captivity, Heckel Discus, for example, that there is virtually nothing most of us can do to make it any easier.
When only a handful of people over the past 50 years have managed to breed them, the reasons why are surely more complex than the exact pH and hardness of their water.
I have had similar experiences with all the plecos I have bred.
It can be paradoxical at times because if you do understand the basics of aquarium water chemistry you can get better results by making up your own designer water but unless you have a system or the knowledge which allows you to keep your water chemistry predictable, you are usually better off not playing around with the water's chemistry.
Avid Trout fly fisherman. ·´¯`·...¸><)))º>




